Project Scope
Clinicians make treatment decisions based on concentrations of bilirubin in an infant’s body. The two relevant measurements of bilirubin concentration are: 1) Transcutaneous Bilirubin (TcB), which measures the accumulation of unconjugated bilirubin in the dermis; and 2) Total Serum Bilirubin (TSB), which measures the level of bilirubin (conjugated or unconjugated) in the bloodstream. Both of these metrics are reported in milligrams/deciliter or micromoles/liter (Subcommittee on Hyperbilirubinemia, 2004). TSB is the measure used in making clinical decisions. Even though TcB underestimates serum bilirubin concentration, since the results of both tests are linearly correlated (with R2 values of 0.86 and 0.85 for the BiliCheck and JM-103, respectively), TcB can be used to accurately estimate TSB once the negative bias has been corrected for (Rodriguez-Capote, Kim, Paes, Turner, & Grey, 2009).
In a recent study, Bhutani and his colleagues assessed that one in seven hundred newborns will have a TcB concentration of 25 mg/dL or more (Watchko, 2006). In healthy full-term newborns, this maximum value will not occur until 48 to 72 hours after birth. Unfortunately, in most public sector hospitals infants delivered vaginally are discharged as soon as the infant and mother are stable (i.e. the infant must urinate, have stable vital signs, and show no problems with breastfeeding). The mean length of stay for vaginally birthed newborns is 1.1 days, and all were discharged before 48 hours (Habib, 2013). This means that peak bilirubin levels do not occur under a physicians’ watch (see Figure 1). Infants sent home with mild cases of jaundice may run the risk of developing potentially devastating, permanent effects if they do develop hyperbilirubinemia (Lee, 2011). Furthermore, recent study of infants returned to the hospital after early discharge (early meaning before 48 hours) shows that 84.6% of these returned infants were re-hospitalized due to hyperbilirubinemia (Farhat, 2011). Our aim is to construct a device capable of monitoring trends in bilirubin concentration over the course of treatment during home care. This device would be appropriate for an infant population whose TcB levels range from as low as 7 mg/dL (the low-risk threshold/40th percentile for term infants at a discharge time of 48 hours) to as high as 17 mg/dL (the high-risk threshold/95th percentile at 84 hours) (Lauer et al.). Infants in the high-risk zone of the nomogram should be kept in the hospital.
Figure 1. Establishing “risk zones” for the prediction of hyperbilirubinemia in newborns. This nomogram is based on hour-specific bilirubin values obtained from 2,840 well newborns >36 weeks gestational age whose birthweights were >2,000 g or >35 weeks gestational age whose birthweights were >2,500 g. The serum bilirubin concentration was measured before discharge. The risk zone in which the value fell predicted the likelihood of a subsequent bilirubin level exceeding the 95th percentile. Obtained from Hyperbilirubinemia in the Newborn, Lauer et.al.
This monitor will determine if the infant’s bilirubin levels are safely below the low-risk threshold or are above it, and will warn the family when the infant is at risk of significant hyperbilirubinemia. Infants with milder cases of jaundice are often sent home without phototherapy, and this monitor will warn parents if these infants need to be returned.
Specific Design Requirements
· Comfort of infant:
o Weight of those parts of the device resting directly on infant must not exceed 4 oz.
§ Comparable to a typical smartphone.
o There should be minimal irritation to the infant’s skin or eyes
o There should be minimal risk of injury: pinching hazards, electrocution, contusions, burns, choking, etc.
§ There should be no risk of death from choking. According to the Consumer Product Safety Commission a device with a diameter of 1.75 inches (44.4mm) or less that is intended for use by children younger than 3 years of age is banned. The design must adhere to this guideline for any associated loose parts.
· Portability:
o Device must fit inside a 20 liter volume.
§ Size of small-medium backpack
o Total weight of device must be less than 10 pounds.
· Ease of use:
o A caretaker who lacks extensive technical background can use device with minimal operator training
· Range:
o The device should detect serum bilirubin levels as low as 7 mg/dL and, ideally, as high as 17 mg/dL (Error! Reference source not found.).
· Reliability:
o Measurement reliability should be comparable to current screening tools (bilirubinometers profiled in Romagnoli et al.):
§ Sensitivity of 80% or better
§ Specificity of 80% or better
§ Positive predictive value of 15% or better
§ Negative predictive value of 98% or better
o The device will minimize risk of operator error by providing prompts in the user’s own language.
o The measurement should not be strongly affected by variation in skin color due to melanin or hemoglobin
· Power:
o The device will be battery-operated. Specifically, it will be powered by a laptop battery, approximately 60W-hrs (Dell 4-Celll Battery)
o Total energy requirement for 3 days is approximately 60 W-hrs.
-
Software:
o An algorithm will compare estimated serum bilirubin to the nomogram (either automatically or through operator action) (see Error! Reference source not found.)
o This will trigger an alert if the infant exceeds preset risk threshold.
§ Warning threshold could use standard nomogram values, or could be customized by the physician
· Time to Perform a Measurement:
o It should not take more than 5 minutes for the infant’s caretaker to finish a measurement
§ Comparable to common care activities such as diaper changing.
· Maximum Time Between Measurements:
o Reading at least once every 8-12 hours (Subcommittee on Hyperbilirubinemia, 2004), preferably more often.
· Proposed Price Point
o The proposed retail cost is around $100 (comparable to a pulse oximeter).
Look at the following reports for more details about our design process!
Preliminary Report Progress Report
Final Report Design Safe Analysis
Design Schedule
Design



I'm a description. Click to edit me


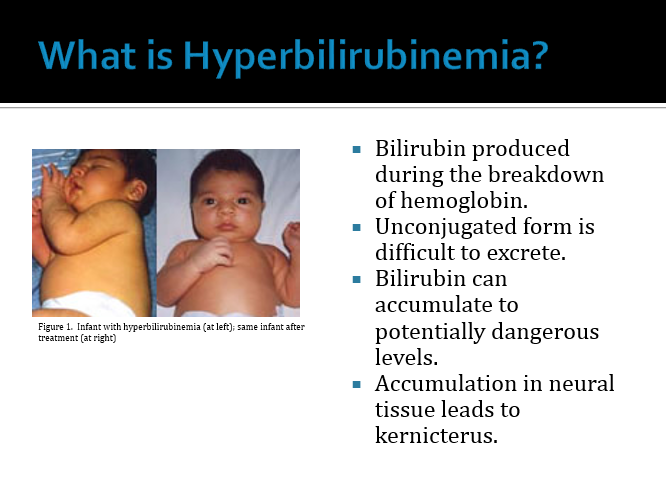






#1
Preliminary Presentation
#2
Progress Presentation
#3
Final Presentation


